Legionella detection in hot water distribution systems of closed community and tourist accommodation facilities in the Lazio Region, Italy: risk assessment and prevention
Gian Loreto D’Alò1, Alessandra Messina1, Cinzia Mozzetti1, Patrizia De Filippis1
1Section of Hygiene, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”
Introduction
Some bacteria belonging to the genus Legionella, mostly Legionella pneumophila, are recognised as emerging water-borne pathogens in developed countries, able to cause two different human infections: Legionnaires’ disease (LD), a community- or hospital-acquired, severe and potentially fatal atypical pneumonia,1 and Pontiac Fever (PF), a febrile and generally benign non-pneumonic disease.2 The underlying mechanisms responsible for either PF or LD have not yet been elucidated.3,4
What is known, however, is that chronic lung disease, diabetes and various conditions associated with immunodeficiency and also increasing age, male sex and smoking are all important risk factors for LD.5,6
All infections are acquired through inhalation of aerosols or aspiration of water containing Legionella,7 and there is no evidence of person-to-person transmission: only one case has been described recently, although the scientific community is waiting for further evidence to confirm this.8,9
Legionella bacteria grow naturally in freshwater environments, but find optimal growth conditions in engineered water system, such as cooling towers, plumbing systems, water tanks, decorative fountains, whirlpool spas, mist machines, dental-unit water, hospital equipment and showerheads.7,10-13 In these environments, many factors, such as aged plumbing, low flow rate or stagnation of the water, dead legs, surface materials and roughness, but also the chemical constituents of the water, warm temperatures and poor management, can promote the proliferation of Legionella. These conditions have been found to be conducive to the formation of biofilms, which often leads to the establishment and maintenance of chronic water system colonisation by Legionella.14,15,16 Furthermore, the capacity of these bacteria to live in free-living protozoa such as amoebae, their natural host, can protect them from adverse conditions or biocide treatments.17-22
Several decontamination and/or disinfection techniques are available to control the risk of Legionella infections.
Decontamination treatments include:
- heat shock (maintaining temperatures 70°C – 80°C for three full days within the water system);23
- hyperchlorination (injecting chlorine into the plant to reach free residual chlorine concentrations of 20-50 mg/L throughout the whole water system).23
Non-continuous disinfection treatments include:
- non-continuous heat treatment (maintenance of a temperature of 55°C – 60°C23);
- chlorine dioxide, that has been shown to be effective if concentrations are > 0.5mg/L;24-28
- UV light;29,30
- Copper-silver ionisation, that has been shown to be effective if used in combination with free chlorine or chlorine dioxide;31-34
- Hydrogen peroxide and silver. Evidence of the efficacy of this treatment is taken mostly from in-vitro studies;35,36
- Monochloramine;37-40
- Ozone;41
- Peracetic acid.41-46
However, given the complexity of the environments in water systems, no ‘gold standard’ exists, and all the existing techniques have advantages and disadvantages.
Although L. pneumophila infections are still generally underestimated, notified cases have increased over the years and the most frequent source of infection is contaminated water from distribution systems.
There are numerous studies that have shown how the water supplies in hospitals have been directly linked to the occurrence of hospital-acquired legionellosis.47,48,49,50
There is also evidence of the widespread presence of Legionella in hot water distribution systems in hotels, schools, sport facilities, offices and private residences,17,51-55 responsible for sporadic and community-acquired cases of LD.6 The mortality rate for cases is much higher in health facilities than in the community, and these data are not surprising, as people affected by healthcare-associated legionellosis are probably more likely to suffer from underlying conditions.56,57
The number of LD cases in the United States reported by the Centers for Disease Control and Prevention (CDC) has been on the rise since 2000. Although a total of 6,000 cases of LD were reported in 2015, the true incidence may still be underestimated.58
The most recent ECDC ‘Annual Epidemiological Report on Legionnaires’ disease’, based on data for 2016 retrieved from the European Surveillance System, reported 7,069 cases of LD, of which 6,560 (92.8%) were classified as confirmed. As in 2015, the number of notifications per 100,000 inhabitants was 1.4, which remains the highest number recorded. Of 5,404 cases with known outcomes, 441 were reported to have been fatal, with a fatality rate of 8.2%. L. pneumophila serogroup 1 was the most commonly identified pathogen. Of all environmental sites testing positive, 411 were water systems, 22 cooling towers and 13 pools.59
Four countries (France, Germany, Italy, and Spain) accounted for 69% of all notified cases, and Italy was the country with the largest number.59
In fact, in the last ten years, the annual number of cases of legionellosis, primarily LD notified to the Italian National Surveillance System rose from 192 to 2,014, with an incidence of 33.2/1,000,000 cases/people-year.57,60 In 2017, 78.5% of the LD cases reported were community-acquired, whereas 11.9% were travel-associated, 6.2% were nosocomial and 3.0% involved persons living in nursing homes, rehabilitation facilities or retirement homes. The case-fatality rate was 10.1% for community-acquired cases and 51.1% for hospital-acquired cases.57
In 2000, the Italian Institute of Health (ISS) produced the first guidelines on the control and prevention of legionellosis,61 followed, in 2005, by instructions for tourist accommodation and spas.62 In May 2015, a new document was approved with the aim of gathering and updating all the instructions reported in the previous national guidelines and regulations and integrating them in a single text.23 The instructions recommend that factors critical for Legionella growth and diffusion must be taken into account during the design and maintenance of water systems. Although it cannot be guaranteed that the bacteria will be completely eradicated, such measures reduce possible contamination.
The aim of this study was to evaluate the frequency of colonisation by Legionella in some hot water systems of different facilities (recreational facilities, retirement homes and group homes) in order to assess possible risks of Legionella infection. The portion of the population using or living in these facilities could potentially be exposed to the risk of infection.
In recent years, the number of people attending sport facilities has greatly increased, with gymnastics courses being organised increasingly often for the elderly and for people undergoing rehabilitative treatments. The individual factors and concomitant diseases affecting these populations can represent a risk for developing legionellosis through the use of contaminated water, especially through showers.53,63-65
Where these were available, we also reported the results of the treatments carried out in some of the structures that tested positive for Legionella to eliminate or contain this contamination.
Finally, we investigated the relationship between the presence of Legionella and heterotrophic plate count (HPC at 22°C and 37°C), parameters indicating general water quality within distribution systems,9,66 to analyse the usefulness of these parameters in predicting the risk of Legionella contamination in hot water systems.
Materials and methods
Collection of samples
To recruit the structures, we were interested in for the survey, an information campaign under undertaken on the problems related to the presence of Legionella in water systems, and free checks on the terminal points of some water systems were offered. The samples were collected from the water systems of those structures whose administrators signed up to the initiative.
From May 2014 to June 2017, water samples were collected from hot water distribution systems of 36 recreational centres, 28 retirement homes and nine group homes located in the area around Rome (Lazio, Italy). All these buildings were supplied from the public network using groundwater. The water supplied by the municipality through the water network is drinkable, and this water is used by the facilities for all normal domestic uses. None of the facilities had a well connected to the domestic water system, and water gathered from wells was not therefore mixed with drinkable water and was used only for purposes other than domestic use (i.e. irrigation). All water samples were collected from showerheads.
Sampling method
All samples were taken without previously running the water and without flaming the outlet point, in accordance with the Italian Guidelines for water sampling in common use conditions, namely ‘instantaneous sampling’, to simulate theoretical user exposure.23 The water temperature was also measured during sampling, and although all samples were collected by turning on the hot water, some of the samples did not exceed 30°C. Legionella standard sampling procedures for water were those provided in ISO 11731:1998 (Water quality – detection and enumeration of Legionella) and in the Italian guidelines for the prevention and control of legionellosis.23
The samples were collected in 1L sterile polyethylene bottles with 10% sodium thiosulphate to neutralise the chlorine (able to neutralise up to 5 mg/L of residual free and combined chlorine).67,68
The samples were transported to the laboratory in suitable containers, at room temperature and protected from direct light, for microbiological analysis.
Microbiological analysis
Legionella isolation was performed in accordance with ISO 11731:199869 and the Italian guidelines with minor modifications,23 as described in our previous work.65,70 The water sample was filtered through a 0.20 μm pore-sized cellulose nitrate filter (Sartorius). The filter was resuspended in 5 mL of the original water sample and shaken using a vortex for 2 minutes to detach the bacteria. In order to reduce contamination by interfering microorganisms, the sample was held at 50°C for 30 minutes. An aliquot of 0.5 mL was then applied to plates of Legionella CYE agar base (Oxoid) with the addition of BCYE growth supplement and GVPC selective supplement (Oxoid). The inoculated plates were incubated at 37±1°C in 2.5% CO2 for ten days and read every day.
Suspected colonies were counted from each sampling plate. We then selected at least five suspected colonies, where available, for each plate,23 and we subsequently confirmed Legionella positivity by their inability to grow on CYE agar base without BCYE growth supplement. Finally, we evaluated each suspected Legionella colony by final agglutination using a Legionella Latex Test Kit (Oxoid).71
The test allows a separate identification of L. pneumophila serogroup 1 and L. pneumophila serogroup 2-14, and detection of seven Legionella (polyvalent) species, which have been implicated in human disease: L. longbeachae, L. bozemanii 1 and 2, L. dumoffii, L. gormanii, L. jordanis, L. micdadei, and L. anisa. Positive and negative control for Legionella, performed each time, was applied.
All positive colonies and a subset of negative colonies were also confirmed by PCR (see paragraph 2.4).
The results were expressed in Colony Forming Units per litre (CFU/L), and the detection limit, based on the concentration factor and the volume of the inoculum, was 10 CFU/L. The accuracy of the method was checked each month using internal titered controls.
An aliquot of each water sample was also taken to determine the load of the Heterotrophic Plate Count (HPC) at 22°C and 37°C. These bacteria were determined in duplicate using the pour plate method, with standard Plate Count Agar (Oxoid). The results are expressed in CFU/mL.67,72
PCR testing
All colonies that tested positive in the Legionella latex test and colonies showing morphological characteristics similar to those of Legionella, growing only on selective medium, but negative in the agglutination test, were also confirmed using polymerase chain reaction (PCR) assay, according to the Van der Zee et al. protocol.73 The primer set used, LEG1 (50TACCTACCCTTGACATACAGTG-30) and LEG2 (50-CTTCCTCCGGTTTGTCAC-30), was derived from the 16S rRNA gene sequence and used to amplify a 200 bp DNA fragment specific for all Legionella species. The PCR reaction mixture, 25 μL final volume, contained 10 pmol of each primer, LEG1 and LEG 2, 200 μM of each dNTP, 3 mM MgCl2, and 2 U AmpliTaq Gold polymerase in 1 × PCR buffer (Promega). Samples were preheated for 10 minutes at 95 WC, followed by 40 cycles of 30 seconds at 94 WC, 30 seconds at 60 WC, and 30 seconds at 72 WC, with a final extension of 5 minutes at 72 WC. A negative and positive control was included in each PCR run. Amplified DNA was detected using agarose gel electrophoresis and ethidium bromide staining.
All colonies negative in the agglutination test were also negative for PCR.
Statistical analysis
All statistical tests were 2-sided, with statistical significance set at 0.05. Continuous variables were summarised using descriptive statistics and expressed as average and standard deviation (SD), as appropriate. Comparison between two means was undertaken using Student’s t-test. Categorical data were expressed in percentages or summarised in contingency tables.
When necessary, continuous variables were categorised as follows:
- Legionella load was categorised for descriptive analyses in three group, based on the implications for the advices to be given on the decontamination procedures:23 Legionella <102 CFU/L; Legionella ≥ 103 CFU/L but < 104 CFU/L; Legionella ≥ 104 CFU/L. Given the paucity of data, only two Legionella load categories were considered for statistical analyses and calculation of Odds Ratios (OR) and 95% confidence intervals (CI), when assessing decontamination treatments’ effectiveness: Legionella <102 CFU/L; Legionella ≥ 103 CFU/L;
- both HPCs at 22°C and at 37°C were categorised in five groups (HPC ≤ 10 CFU/mL; 10 CFU/mL < HPC ≤ 100 CFU/mL; 100 CFU/mL < HPC ≤ 300 CFU/mL; 300 CFU/mL < HPC ≤ 500 CFU/mL; HPC > 500 CFU/mL), consistently with our previous works,65,70 in order to verify our hypotheses about a possible correlation between certain HPCs values and the presence/absence of Legionella. These HPCs groups were used both for descriptive analyses and for the construction of contingency tables.
The qualitative analysis of categorical data was performed by constructing contingency tables and applying Fisher’s exact test or Chi-square test, where appropriate.
Wilcoxon-Mann-Whitney tests were performed in order to compare concentrations in two populations: precisely, this statistical test was used to compare the following pairs of variables: HPCs at 22°C and at 37°C; L. pneumophila sg 1 and L. pneumophila sg 2-14; L. pneumophila sg 1 and Legionella spp.; L. pneumophila sg 2-14 and Legionella spp.
Statistical analysis was performed using STATA version 13.74
Results
Legionella prevalence
A total of 370 water samples were collected, from 370 sampling points (showerheads), from hot water distribution systems in 73 buildings, during 93 sampling operations performed from May 2014 to June 2017. The collected samples are divided as follows:
- 216 samples from 36 recreational and tourist accommodation facilities. Specifically, 17 were sports centres, eight campsites, five hotels, three bathing establishments, two holiday farms and one amusement park.
- 154 samples from 37 social-assistance structures. Specifically, 28 were retirement homes and nine group homes.
On average, 3.98 samples (SD=2.75) were collected during each sampling procedure, based on the number of showers provided with hot water in the facilities.
Legionella was found in 97 samples (26.2%) collected from 21 (28.8%) of the structures inspected. Specifically:
- 39 samples were found to be positive for L. pneumophila sg 1 alone;
- 38 samples were found to be positive for L. pneumophila sg 2-14 alone;
- 13 samples were found to be positive for Legionella spp alone;
- five samples were found to be positive for both L. pneumophila sg 1 and sg 2-14;
- one sample was found to be positive for both L. pneumophila sg 1 and Legionella spp;
- one sample was found to be positive for both L. pneumophila sg 1 and sg 2-14 and Legionella spp.
With regard to the structures, Legionella was found in the water systems of 12 (33.3%) recreational and tourist accommodation facilities and nine (24.3%) social-assistance facilities, respectively. However, this difference was not statistically significant in the Fisher’s exact test (p = 0.4459).
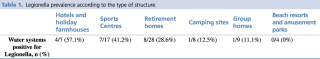
Legionella prevalence according to the type of structure is shown in detail in Table 1.
Table 1 - Legionella prevalence according to the type of structure.
Legionella load
Overall, in 53 (54.6%) samples, Legionella load was ≥ 103 CFU/L, while in 27 (27.8%) it was ≥ 104 CFU/L.
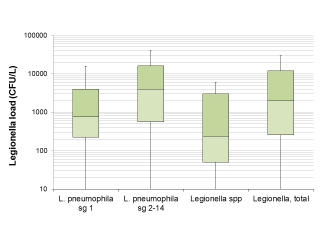
The median Legionella load in the positive samples was 2,000 CFU/L (IQR: 260-12,000 CFU/L). Regarding the differences between isolated serogroups, Legionella load in samples positive for L. pneumophila sg 2-14 alone appeared significantly higher in the Wilcoxon-Mann-Whitney test compared to the samples positive for L. pneumophila sg 1 alone (p = 0.0147) and for Legionella spp. (p=0.0013). The difference in Legionella load between samples positive for L. pneumophila sg 1 and Legionella spp., however, was not significant (p = 0.1236) (see also Figure 1).
Figure 1 - Legionella load in samples found positive for Legionella based on the serogroup or species.
General water quality: Heterotrophic Plate Counts (HPCs)
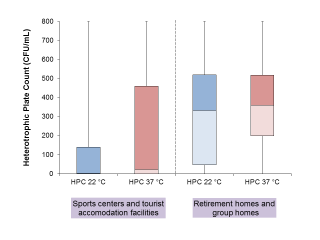
Data on HPC at 22°C and 37°C are available for 291 samples belonging to 66 water systems, with 145 samples collected from 31 sports centres or tourist accommodation facilities, and 146 from 35 social assistance facilities. Samples collected from the latter facilities were significantly more contaminated in the Wilcoxon-Mann-Whitney tests, both for HPC at 22°C (p < 0.00001) and at 37°C (p < 0.00001) (see also Figure 2).
Figure 2 - Heterotrophic Plate Counts (HPCs) at 22 °C and at 37 °C observed in Sports centers/Tourist accommodation facilities a
Heterotrophic Plate Counts (HPCs) related to Legionella presence
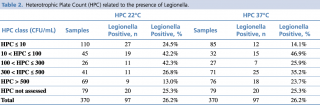
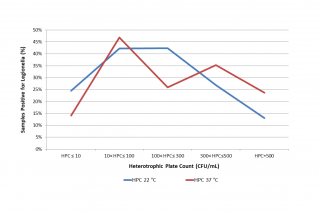
The distribution of Legionella presence appears to be significantly correlated to both HPC at 22°C (p = 0.0035) and HPC at 37°C (p = 0.0023) using Chi-square tests (see Table 2 and Figure 3). It would seem that intermediate values of HPC at 22°C (10 < HPC ≤ 300) favour the presence of Legionella, while very high HPC 22°C (> 500 CFU/mL) have a deterrent effect. Regarding HPC at 37°C, on the other hand, data show that very low HPCs (< 10 CFU/mL) correlate negatively with the presence of Legionella, while medium-low (10 < HPC ≤ 100) or medium-high HPC (300 < HPC ≤ 500) correlate positively.
Table 2 - Heterotrophic Plate Count (HPC) related to the presence of Legionella.
Figure 3 - Percentage of Legionella positive samples related to Heterotrophic Plate Count (HPC, CFU/mL) at 22 °C and 37 °C.
Repeated samplings
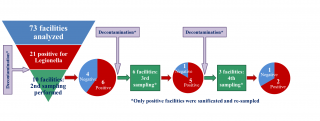
At least a second sampling was performed in seven out of 12 (58.3%) sports centres or tourist accommodation facilities, and in two out of nine social-assistance facilities positive for the presence of Legionella during the first sampling. The second and any subsequent sampling operations were performed in all cases following decontamination intervention, carried out at the initiative of the managers of the structures to eliminate Legionella from the water system. In one of the structures, following post-decontamination negativisation, a new sampling operation (third sampling), which again revealed the presence of Legionella, was carried out after one year. The structure was therefore resampled a fourth, a fifth and a sixth time: taking into account the extensive period between negativisation and the third sampling, the sampling operations following the first negativisation were considered in the analyses as the first second and third sampling operations, respectively. Ultimately, ten, six and three structures were subjected to second, third and fourth sampling operations, respectively.
Overall, in 60% (6/10) of the structures analysed, Legionella was still present on the second sampling operation following intervention to disinfect the water system. In 83% (5/6) of the structures in which a third sampling operation was undertaken, Legionella was still present, while 67% (2/3) of the structures were also positive for the presence of the bacterium at the fourth sampling (Figure 4).
Figure 4 - Repeated water systems’ samplings and Legionella persistence
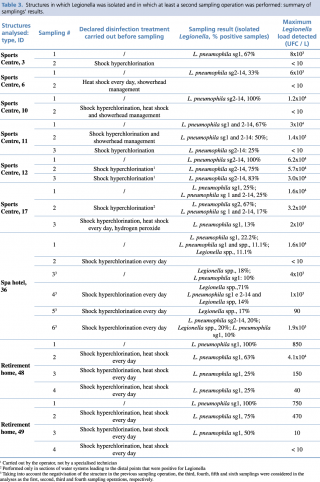
In five (55.5%) of the resampled structures, a Legionella load equal to or greater than 103 CFU/L was found in at least one sample after disinfection, and in four of these (44.4%), the concentration was even higher than 104 CFU/L. In all cases but one (SPA Hotel, ID 36 in Table 3), the Legionella class of serogroups or species identified in the sampling operations performed after disinfection was the same one identified during the first sampling operation (Table 3).
Table 3 - Structures in which Legionella was isolated and in which at least a second sampling operation was performed: summary of samplings’ results.
Legionella persistence after water system disinfection
We performed 19 sampling operations in the facilities in which Legionella was found a few days after water system disinfection had been carried out.
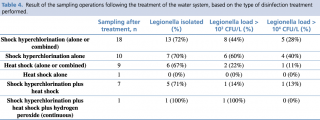
With regard to the treatments carried out before sampling, in ten cases only shock hyperchlorination was performed, in seven cases this treatment was combined with heat shock, and in one case the use of hydrogen peroxide was combined with the above two treatments. Finally, in only one case, the manager relied solely on the heat shock method (Table 3 and Table 4).
Table 4 - Result of the sampling operations following the treatment of the water system, based on the type of disinfection treatment performed.
Given that maintenance of the terminals (showerheads) should be a routine hygiene practice, even if declared by the operator during sampling as a decontaminating method implemented, this process has not been included in the analysis as a ‘combined treatment’ if associated with one of the abovementioned methods.
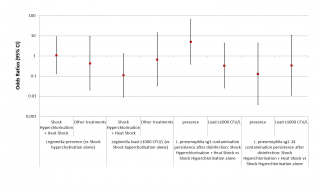
Given the limited number of sampling operations performed after disinfection, and the almost total overlap between the cases in which the shock hyperchlorination was performed in combination and those in which this combination consisted of the addition of the process to heat shock, we decided to analyse only the difference in the efficacy of shock hyperchlorination alone vs shock hyperchlorination combined with heat shock, or of other treatments vs shock hyperchlorination alone. Although the data seem to indicate a greater efficacy of the combination of shock hyperchlorination and heat shock in maintaining the Legionella load < 103 CFU/L, this correlation does not reach statistical significance (p = 0.081) (Figure 5, third column). Moreover, the addition of heat shock to shock hyperchlorination does not seem to have any effect on the presence of Legionella in the water system (p = 0.949) (see Figure 5, first column). We have also tried to establish a correlation between Legionella serogroup and disinfection efficacy, but, although the data seem to indicate a greater efficacy of the combined treatments against L. pneumophila sg 2-14 compared to Legionella sg 1, at least in terms of the presence of the bacterium in the water system, the results are never statistically significant.
Figure 5 - ORs for the presence and the load of Legionella based on the disinfection treatments carried out
Discussion
We found a high prevalence of Legionella in the water systems of the buildings analysed. This prevalence was higher among tourist accommodation facilities than retirement homes and group homes, but Legionella prevalence was highest in hotels, holiday farmhouses and sports centres (57.1% to 41.2%) and lowest in camping sites, group homes, beach resorts and amusement parks (12.5% to 0%) (Table 1).
Legionella prevalence in the water systems of hotels
Bonetta et al.78 found a significant difference between isolation of Legionella in hotel water systems using the culture method (42% water systems positivity, 100 CFU/L detection limit) and using RT-PCR (74%). A similar difference (10% vs 38%) was found by Edagawa et al. in hotels,79 and by Collins et al. (6% vs 48%) in households.80 However, this difference could be due to viable but non-culturable cells (VBNC) of L. pneumophila, which are detectable only using sensitive molecular techniques81 and whose ability to cause LD or PF epidemics is still debated.66,82,83 On the other hand, some authors hypothesise that these forms can become vital and proliferate when the concentration of the disinfectant used to control contamination in the water system falls below a certain threshold value (e.g. monochloramine < 1.5 mg/L).83-85
Legionella prevalence in recreational facilities
Legionella prevalence in domestic water systems
The prevalence of Legionella we have identified in retirement homes and group homes, where water system complexity can be compared to domestic systems, is consistent with previous reports in the literature. In fact, in Italy Legionella sample positivity in the hot water of domestic water systems ranges from 22.6% to 33%, although one study reports a higher prevalence (53.1%) of the bacterium in water systems.51,54,91,92,93 However, further confirmation of our data, with Legionella prevalence between 6% and 35% in home water systems, is provided by studies conducted in the US and other European countries.77,80,94-97
Legionella prevalence and buildings size
As already shown in our previous studies,53,65,70Legionella has been found more frequently in larger buildings. In fact, we found Legionella in 45.8% of the water systems of hotels and sports centres compared to 10% of beach resorts and camping sites.53 Though not statistically significant (Fisher’s exact test: p = 0.0607), these data, combined with data on the correlation in retirement homes and group homes between number of storeys and presence of Legionella (p = 0.0514),65,70 seem to confirm the link already identified in the literature between the complexity of the water system and Legionella colonisation and persistence.52,63,77,95,98
Legionella load
Our data on Legionella load are consistent with the previous literature. In fact, considering studies examining buildings similar to the ones we investigated, the percentage of positive samples with a Legionella load ≥ 103 CFU/L usually ranges from 58% and 83%, while the percentage falls to 9%-38% when considering only samples with a Legionella load ≥ 104 CFU/L.51,54,55,77,78,92,93,99 Considerably lower contamination values were found in three studies conducted in Turkey, Greece and Italy, with only 17%, 11%, and 5%, respectively, of positive samples with a Legionella load ≥ 103 CFU/L.75,76,100
Results for drinking water parameters
Our HPC values are higher when compared to the results of extensive monitoring in warm, in-building distribution systems in Germany101 and, for example, the works by Totaro et al. and Baggiani et al. recently conducted in Italy, in which the HPC ranged from 1 to 400 and had a mean value at the distal point of 41.2 ± 59 CFU/mL, respectively.91,93 Both works show that HPC were significantly lower at the distal point than at the inlet. Bargellini et al. (2001) reported a geometric mean value for HPC at 22°C and at 37°C of 35 and 101 CFU/mL, respectively.99 Conversely, Moutchouri et al. found widespread contamination of hotel water systems, with 72% of samples characterised by an HPC ≥ 400 CFU/mL. They also found a statistically significant (p < 0.001) increase in Legionella prevalence among samples with HPC ≥ 400 CFU/mL,55 and proposed the HPC as an indicator of the presence of Legionella spp. Similarly, Bargellini et al. (2001) showed a significant correlation between values of HPC at 37°C > 150 CFU/mL and presence of Legionella by performing univariate regression (OR 2.31, 95%CI 1.55-3.43), and a significant correlation between values of HPC at 22°C > 27 CFU/mL both by univariate regression (OR 2.68, 95%CI 1.80 to 4.00) and by multiple logistic regression (OR 2.24, 95%CI 1.47 to 3.42).99 Solimini et al. (2014) in their in-vitro experiments showed that the presence of other bacteria, such as the heterotrophs, sustain L. pneumophila growth, being probably the source of other essential nutrients. In fact, although iron was found to be linked to L. pneumophila growth, this metal was unable to enhance L. pneumophila growth without the contemporary presence of other microrganisms.102 In our previous papers65,70 we found a statistically significant peak of Legionella prevalence for HPC at 22°C between 10 and 300 CFU/mL. Yet, despite their relationship with the presence of Legionella, HPC are not considered an indicator of health risk in themselves.103 Accordingly, they are not considered suitable for public health target-setting as part of a Water Safety and Risk Management Plan for a potable water supply.9,104,105 Conversely, monitoring the changes in HPC between water entering a facility and distal points can help in identifying where stagnation in a water distribution system may be occurring, leading to microbial growth. This is the reason why, although there is no precise threshold, the ‘Reference Analytical Methods for Water Intended for Human Consumption According to Italian Legislative Decree No 31/2001’ provide for the mandatory assessment of HPC at 22°C. This must not experience ‘anomalous variations’.67
Efficacy of Legionella disinfection systems
Despite a clear trend indicating greater effectiveness of combining heat shock with shock hyperchlorination, at least in reducing Legionella at high concentrations (> 103 CFU/L) but not on Legionella presence, we did not find any significant correlation between the disinfection treatment applied and the presence/load of Legionella in the water systems analysed.
However, these results could have been due to the limited number of facilities analysed and to the fact that often the same treatment was repeated several times in the same structure, making the data redundant. Another limitation of the study is that few types of disinfection treatment were performed by the building administrator, and the fact that we have no information about non-continuous disinfection treatments carried out before the first sampling operation. Moreover, shock hyperchlorination sometimes carried out by the operator and not by a specialised technician or performed only in sections of water systems leading to the distal points that were positive for Legionella (see Table 3) may have contributed to increasing heterogeneity in the effectiveness of this type of treatment.
Considering the facilities vertically (Figure 4 and Table 3), we can see how frequently the sanitisation interventions performed were ineffective. Indeed, according to the available data, Legionella was still found in the water systems after decontamination in 68% of facilities inspected.
Based on their experience, Marchesi et al. proposed the adoption of electric boilers and chlorine dioxide to prevent and/or reduce Legionella contamination, while monthly shock hyperchlorination and heat shock were considered less effective, and filter installation at the distal point, though effective, was far too expensive and is therefore used primarily to prevent nosocomial infections.106,107 Despite its short-term effectiveness and low cost, heat shock is widely considered in the literature to be a temporary method to reduce Legionella contamination in water systems, because recontamination reoccurs within one to two months after the intervention is performed.106-108 Slightly greater long-term effectiveness is associated with shock hyperchlorination, but only when it is followed by non-continuous water chlorination.108,110 In our work, as in the paper by Marchesi et al.,106 there were no facilities in which a copper-silver ionisation system was installed as a non-continuous disinfection method after decontamination was performed, so we could not establish the effectiveness of this process in reducing Legionella presence as others have done. In particular, Lin et al. found this treatment to be effective both in the short and long term,107-109 while Ortolano et al. found that its efficacy is strongly correlated with the content of dissolved solids in the water.110 At the same time, we did not have any data about the effectiveness of other significant, well studied treatments such as chlorine dioxide and monochloramine, which have frequently been shown to be effective in reducing Legionella contamination.24-28,38-40,111 Recently, Totaro et al. (2019) reported that both chlorine dioxide and anolyte (hypochlorous acid) had been able to avoid Legionella spp. growth in previously contaminated water networks of nursing homes in a long-term period.111
Along with efficacy, safety must be taken into account when choosing the disinfection method to be implemented. Many disinfection methods use disinfectants and can create by-products that can be harmful to human health. For example, copper is the most widely investigated metal because of its role in the formation of amyloid plaques in Alzheimer’s disease, with 36 studies assessing concentrations of this metal in different biological matrices, and the successful treatment of this disorder in rodent models using copper chelating is a very interesting development.112,113 However, the concentrations of copper and silver effective for disinfection are 0.2 to 0.6 mg/L and 0.02 to 0.06 mg/L, respectively.114 In 2004, the WHO established a threshold of 2 mg/L for copper concentrations in drinking water:115 this threshold should permit consumption of 2-3 litres of water per day, without exceeding the tolerable upper intake level of 10 mg/day.116 With regard to chlorination, high residual chlorine could react with organic materials, accelerating the production of trihalomethanes, which are known carcinogens,110 and an increased risk of scalding through heat shock has been reported by some authors.103,117 By-product formation in the presence of bromide (bromate) and chlorine (chlorate) can also occur when ozonation is performed.118 Finally, there are damaging effects on the rubber, lead or copper components of the plant and nitrogen products can be produced in the water following non-continuous disinfection using monochloramine.37
Conclusions
LD cases diagnosed in Italy are increasing slowly but constantly.57 The Italian situation is in line with the picture in Europe as a whole, where legionellosis cases have been increasing since 2011. This increasing trend is probably driven by several factors, such as improved surveillance, travel patterns and climate change, since aging can only partially explain the trend: indeed, the age-standardised notification rate has also increased over the same period. In fact, weather conditions such as temperature, humidity and rainfall have been associated with a higher LD incidence, as they have an effect in terms of increased use of aerosol-producing devices or installations in the environment, such as cooling towers.59 The attention given to Legionella must remain high if we want to prevent epidemic clusters of disease. As evidence of this, we need only look at the most recent epidemic, probably derived from cooling towers in Lombardy, which caused the second largest epidemic of all time in terms of number of cases of Legionnaires’ disease due to pollution of the cooling towers.119,120 The high prevalence of Legionella we have identified, and in general the low quality of water we detected, together with epidemiological data, suggest that there is still significant work to be done to improve the management of the water systems of private facilities. Our work included the efficacy of disinfection systems only as a secondary outcome, and was not powered for evidencing statistically significant differences in the efficacy of the various treatments carried out. More research is warranted to assess the comparative effectiveness of decontamination methods.
References
- Tsai TF, Finn DR, Plikaytis BD, McCauley W, Martin SM, Fraser DW. Legionnaires’ Disease: Clinical Features of the Epidemic in Philadelphia. Ann Intern Med. 1979; 90(4): 509-17. PubMed PMID: 434627.
- Glick TH, Gregg MB, Berman B, Mallison G, Rhodes WW Jr, Kassanoff I. Pontiac Fever. An Epidemic of Unknown Etiology in a Health Department. I. Clinical and Epidemiologic Aspects. Am J Epidemiol. 1978;107(2):149-60. PubMed PMID: 623097.
- Tossa P, Deloge-Abarkan M, Zmirou-Navier D, Hartemann P, Mathieu L. Pontiac Fever: An Operational Definition for Epidemiological Studies. BMC Public Health. 2006; 28(6): 112. PubMed PMID: 16646972; PubMed Central PMCID: PMC1468404.
- Wang C, Saito M, Tanaka T, Amako K, Yoshida S. Comparative Analysis of Virulence Traits Between a Legionella Feeleii Strain Implicated in Pontiac Fever and a Strain That Caused Legionnaires’ Disease. Microb Pathog. 2015; 89: 79-86. doi: 10.1016/j.micpath.2015.09.004. Epub 2015 Sep 18. PubMed PMID: 26386398.
- Phin N, Parry-Ford F, Harrison T, Stagg HR, Zhang N, Kumar K, Lortholary O, Zumla A, Abubakar I. Epidemiology and Clinical Management of Legionnaires’ Disease. Lancet Infect Dis. 2014;14(10):1011-21. doi:10.1016/S1473-3099(14)70713-3. Epub 2014 Jun 23. Review. PubMed PMID: 24970283.
- European Centre for Disease Prevention and Control (ECDC), 2016. Legionnaires’ Disease in Europe, 2014. ECDC, Stockholm, Sweden.
- Barker KA, Whitney EA, Blake S, Berkelman RL. A Review of Guidelines for the Primary Prevention of Legionellosis in Long-Term Care Facilities. J Am Med Dir Assoc. 2015; 16(10):832-6. doi: 10.1016/j.jamda.2015.05.015. Epub 2015 Jul 6. Review. PubMed PMID: 26155722.
- Correia AM, Ferreira JS, Borges V, Nunes A, Gomes B, Capucho R, Gonçalves J, Antunes DM, Almeida S, Mendes A, Guerreiro M, Sampaio DA, Vieira L, Machado J, Simões MJ, Gonçalves P, Gomes JP. Probable Person-To Person Transmission of Legionnaires’ Disease. N. Engl. J. Med. 2016; 374 (5): 497–498. https://doi.org/10.1056/NEJMc1505356.
- World Health Organization, 2017. Guidelines for Drinking-water Quality: Fourth Edition Incorporating the First Addendum. Retrieved from. http://apps.who.int/iris/bitstream/10665/254637/1/9789241549950-eng.pdf?ua=1 , Accessed date: 8 March 2018.
- Atlas RM, Williams JF, Huntington MK. Legionella Contamination of Dental-Unit Waters. Appl Environ Microbiol. 1995; 61(4):1208-13. PubMed PMID: 7747943; PubMed Central PMCID: PMC167375.
- Barrabeig I, Rovira A, Garcia M, Oliva JM, Vilamala A, Ferrer MD, Sabrià M, Domínguez A. Outbreak of Legionnaires’ Disease Associated with a Supermarket Mist Machine. Epidemiol Infect. 2010; 138(12):1823-8. doi: 10.1017/S0950268810000841. Epub 2010 Apr 15. PubMed PMID: 20392306.
- Cowen KA, Ollison WM. Continuous Monitoring of Particle Emissions During Showering. J Air & Waste Manag Assoc. 2006; 56(12):1662-8. PubMed PMID: 17195485.
- Schoen ME, Ashbolt NJ. An In-Premise Model for Legionella Exposure During Showering Events. Water Res. 2011; 45(18):5826-36. doi: 10.1016/j.watres.2011.08.031. Epub 2011 Sep 5. PubMed PMID: 21924754.
- Abdel-Nour M, Duncan C, Low DE, Guyard C. Biofilms: The Stronghold of Legionella pneumophila. Int J Mol Sci. 2013;14(11):21660-75. doi:10.3390/ijms141121660. Review. PubMed PMID: 24185913; PubMed Central PMCID: PMC3856027.
- Declerck, P. Biofilm: The Environmental Playground of Legionella pneumophila Environ. Microbiol. 2010; 12(3):557–566 doi:10.1111/j.1462-2920.2009.02025.x
- Exner, M., Kramer, A., Lajoie, L., Gebel, J., Engelhart, S., Hartemann, P., 2005. Prevention and Control of Health Care-Associated Waterborne Infections in Health Care Facilities. Am. J. Infect. Control 33 (5 Suppl 1): S26–S40. https://doi.org/10.1016/j.ajic.2005.04.002.
- Fields, BS, Benson RF, Besser RE. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002;15(3):506–526. https://doi.org/10.1128/ CMR.15.3.506-526.2002.
- Lau, HY, Ashbolt NJ. The Role of Biofilms and Protozoa in Legionella Pathogenesis: Implications for Drinking Water. J. Appl. Microbiol. 2009; 107 (2): 368–378. https://doi.org/10.1111/j.1365-2672.2009.04208.x.
- Declerck, P., Behets, J., van Hoef, V., Ollevier, F., 2007. Detection of Legionella spp. and Some of Their Amoeba Hosts in Floating Biofilms from Anthropogenic and Natural Aquatic Environments. Water Res. 41 (14):3159–3167. https://doi.org/10.1016/j.watres.2007.04.011
- Cooper IR, White J, Mahenthiralingam E, Hanlon GW. Long-Term Persistence of a Single Legionella pneumophila Strain Possessing the Mip Gene in a Municipal Shower Despite Repeated Cycles of Chlorination. J Hosp Infect. 2008; 70: 154-159 doi:10.1016/j.jhin.2008.06.015
- Cooper IR, Hanlon GW. Resistance of Legionella pneumophila Serotype 1 Biofilms to Chlorine-Based Disinfection. J Hosp Infect. 2010; 74: 152-159. doi:10.1016/j.jhin.2009.07.005
- Simoes LC, Simoes M, Vieira MJ. Influence of the Diversity of Bacterial Isolates from Drinking Water on Resistance of Biofilms to Disinfection. Appl. Environ. Microbiol. 2010; 76 (19): 6673-6679. https://doi.org/10.1128/AEM.00872-10.
- Linee Guida per la prevenzione e il controllo della legionellosi 2015, approvato in Conferenza Stato-Regioni il 7.05.2015. (Italian Guidelines for the Prevention and Control of Legionellosis 2015, approved in State-Regions Conference on 05.07.2015). Available at: http://www.salute.gov.it/imgs/C17pubblicazioni2362allegato.pdf
- Tesauro M, Bianchi A, Consonni M, Pregliasco F, Galli MG. Environmental Surveillance of Legionella pneumophila in Two Italian Hospitals. Ann Ist Super Sanità. 2010; 46 (3): 274-278 doi: 10.4415/Ann_10_03_08
- Scaturro M, Dell’Eva I, Helfer F, Ricci ML. Persistence of the Same Strain of Legionella pneumophila in the Water System of an Italian Hospital for 15 Years. Infect Contr Hosp Epidemiol. 2007; 28 (9): 1089–1092 doi: 10.1086/519869
- Srinivasan A, Bova G, Ross T, Mackie K, Paquette N, Merz W, Perl TM. A 17-month Evaluation of a Chlorine Dioxide Water Treatment System to Control Legionella Species in a Hospital Water. Infect Contr Hosp Epidemiol. 2003; 24 (8): 575-579 doi: 10.1086/502254
- Zhang Z, McCann C, Stout J. E, Piesczynski S, Hawks R, Vidic R, Yu VL. Safety and Efficacy of Chlorine Dioxide for Legionella Control in a Hospital Water System. Infect Contr Hosp Epidemiol. 2007; 28 (8): 1009-1012 doi: 10.1086/518847
- Hoseina IK, Hilla DW, Tan TY, Butchartc EG, Wilsond K, Finlaye G, Burgeb S, Ribeirob CD. Point-Of-Care Controls for Nosocomial Legionellosis Combined with Chlorine Dioxide Potable Water Decontamination: A Two-Year Survey at a Welsh Teaching Hospital. J Hosp Infect. 2005; 61: 100–106 doi:10.1016/j.jhin.2005.02.008
- Kim BR, Anderson JE, Mueller SA, Gaines WA, Kendall AM. Literature Review – Efficacy of Various Disinfectants Against Legionella in Water Systems. Water Res. 2002; 36: 4433-4444. https://doi.org/10.1016/S0043-1354(02)00188-4
- Westerlaken, M. Biological Mechanism behind Legionella Control. Report number P-UB-2006-05. Science Shop for Biology. 2006. Utrecht University, The Netherlands. http://dspace.library.uu.nl/handle/1874/44805 (last accessed September 2016).
- Pedro-Botet ML, Sanchez I, Sabria M, Sopena N, Mateu L, García-Núñez M, Rey-Joly C. Impact of Copper and Silver Ionization on Fungal Colonization of the Water Supply in Health Care Centers: Implications for Immunocompromised Patients. Clin. Infect. Dis. 2007; 45: 84-86. http://cid.oxfordjournals.org/content/45/1/84.short (last accessed September 2016).
- Pianetti A, Sabatini L, Citterio B, Sisti E, Pierfelici L, Bruscolini F. Inactivation of Legionella pneumophila by Combined Systems of Copper and Silver Ions and Free Chlorine. Ig Sanità Pubblica. 2008; 64 (1) 27-40.
- Bedford, B. Legionella Control in Water Systems Using Copper and Silver Ion Generation Systems. Institute of Bioscience and Technology. PhD thesis. 2012 https://dspace.lib.cranfield.ac.uk/bitstream/1826/7983/1/Birgitta_Bedford_Thesis_2012.pdf (last accessed September 2016).
- Casari E, Ferrario A, Montanelli A. Prolonged Effect of Two Combined Methods for Legionella Disinfection in a Hospital Water System. Ann Ig. 2007; 19 (6): 525-532
- Garcia MT, Pelaz C. Effectiveness of Disinfectants Used in Cooling Towers Against Legionella pneumophila. Chemotherapy. 2008; 54: 107-116 doi: 10.1159/000118662
- Sanli-Yurudu NO, Kimiran-Erdem A, Arsian-Aydogdu EO, Zeybek Z, Gurun S. Efficacy of Colloidal Silver-Hydrogen Peroxide and 2-Bromo-2-nitroporopane-1,3-diol Compounds Against Different Serogroups of Legionella pneumophila Strains. Indian J Microbiology. 2012; 52(1): 54-59 DOI 10.1007/s12088-011-0189-z
- Cagarelli R. I metodi di sanificazione e bonifica: efficacia, vantaggi e svantaggi. Servizio di sanità pubblica, Regione Emilia-Romagna 2011
- Flannery B, Gelling LB, Vugia DJ, Weintraub JM, Salerno JJ, Conroy MJ, Stevens VA, Rose CE, Moore MR, Fields BS, Besser RE. Reducing Legionella Colonization in Water Systems with Monochloramine. Emerg Infect Dis. 2006; 12 (4): 588-596 doi: 10.3201/eid1204.051101
- Heffelfinger JD, Kool JL, Fridkin S, Fraser VJ, Hageman J, Carpenter J, Whitney CG. Risk of Hospital-Acquired Legionnaires’ Disease in Cities Using Monochloramine Versus Other Water Disinfectants. Infect Control Hosp Epidemiol. 2003; 24 (8): 569-574. doi: 10.1086/502256
- Kool JL, Carpenter JC, Fields BS. Effect of Monochloramine Disinfection of Municipal Drinking Water on Risk of Nosocomial Legionnaires’ Disease. Lancet. 1999; 353 (9149): 272-277.
- Blanc DS, Carrara Ph, Zanetti G, Francioli P. Water Disinfection with Ozone, Copper and Silver Ions, and Temperature Increase to Control Legionella: Seven Years of Experience in a University Teaching Hospital. J Hosp Infect. 2005; 60 (1): 69-72. doi: 10.1016/j.jhin.2004.10.016
- Conseil Superieur d’Hygiene Publique de France. Gestion du risqué lié au Légionelles. 2001
- Dallolio L, Scuderi A, Rini MS, Valente S, Farruggia P, Bucci Sabattini MA, Pasquinelli G, Acacci A, Roncarati G, Leoni E. Effect of Different Disinfection Protocols on Microbial and Biofilm Contamination of Dental Unit Waterlines in Community Dental Practices. Int. J. Environ. Res. Public Health. 2014; 11(2): 2064-2076 doi:10.3390/ijerph110202064
- Ditommaso S, Biasin C, Giacomuzzi M, Zotti CM, Cavanna A, Ruggenini Moiraghi A. Peracetic Acid in the Disinfection of a Hospital Water System Contaminated with Legionella Species. Infect Control Hosp Epidemiol. 2005; 26 (5): 490-493. DOI: 10.1086/502573
- Farhat M, Trouilhé MC, Foret C, Hater W, Moletta-Denat M, Robine E, Frère J. Chemical Disinfection of Legionella in Hot Water Systems Biofilm: A Pilot-Scale 1 Study; Water Sci Technol. 2011; 64 (3): 708-714 doi: 10.2166/wst.2011.696
- Leoni E., Sacchetti R., Zanetti F, Legnani PP. Control of Legionella pneumophila Contamination in a Respiratory Hydrotherapy System with Sulfurous Spa Water. Infect Control Hosp Epidemiol. 2006; 27(7): 716-721. doi: 10.1086/504364
- D’Alessandro D, Fabiani M, Cerquetani F, Orsi G.B. Trend of Legionella Colonization in Hospital Water Supply. Ann. Ig. 2015;27 (2):460–466. https://doi.org/10.7416/ai.2015.2032.
- Laganà, P, Caruso G, Piccione D, Gioffrè ME, Pino R, Delia S. Legionella spp., Amoebae and Not-Fermenting Gram Negative Bacteria in an Italian University Hospital Water System. Ann Agric Environ Med. 2014; 21 (3): 489-493. doi: 10.5604/12321966.1120623.
- Montagna MT, Cristina ML, De Giglio O, Spagnolo AM, Napoli C, Cannova L, Deriu MG, Delia SA, Giuliano A, Guida M, Laganà P, Liguori G, Mura I, Pennino F, Rossini A, Tardivo S, Torre I, Torregrossa MV, Villafrate MR, Albertini R, Pasquarella C. Serological and Molecular Identification of Legionella Spp. Isolated from Water and Surrounding Air Samples in Italian Healthcare Facilities. Environ Res. 2016; 146:47-50. doi: 10.1016/j.envres.2015.12.015. Epub 2015 Dec 21. PubMed PMID: 26717079.
- Stout JE, Yu VL. Environmental Culturing for Legionella: Can We Build a Better Mouse Trap? Am J Infect Control. 2010; 38(5):341-343. doi: 10.1016/j.ajic.2010.02.002. PubMed PMID: 20569848.
- Borella P, Montagna M.T, Romano-Spica V, Stampi S, Stancanelli G, Triassi M, Neglia R, Marchesi I, Fantuzzi G, Tatò D, Napoli C, Quaranta G, Laurenti P, Leoni E, De Luca G, Ossi C, Moro M, Ribera D’Alcalà G. Legionella Infection Risk from Domestic Hot Water. Emerg. Infect. Dis. 2004; 10 (3):457-464. https://doi.org/10.3201/eid1003.020707.
- Borella P, Montagna MT, Stampi S, Stancanelli G, Romano-Spica V, Triassi M, Marchesi I, Bargellini A, Tatò D, Napoli C, Zanetti F, Leoni E, Moro M, Scaltriti S, Ribera D’Alcalà G, Santarpia R, Boccia S. Legionella Contamination in Hot Water of Italian Hotels. Appl Environ Microbiol. 2005;71(10):5805-13. doi:10.1128/AEM.71.10.5805–5813.2005 PubMed PMID: 16204491; PubMed Central PMCID: PMC1265926.
- De Filippis P, Mozzetti C, Amicosante M, D’Alò GL, Messina A, Varrenti D, Giammattei R, Di Giorgio F, Corradi S, D’Auria A, Fraietta R, Gabrieli R. Occurrence of Legionella in Showers at Recreational Facilities. J Water Health. 2017;15(3):402-409. doi: 10.2166/wh.2017.296. PubMed PMID: 28598344.
- Leoni E, De Luca G, Legnani PP, Sacchetti R, Stampi S, Zanetti, F. Legionella Waterline Colonization: Detection of Legionella Species in Domestic, Hotel and Hospital Hot Water Systems. J. Appl. Microbiol. 2005;98 (2):373-379. https://doi.org/10.1111/j.1365-2672.2004.02458.x.
- Mouchtouri V, Velonakis E, Tsakalof A, Kapoula C, Goutziana G, Vatopoulos A, Kremastinou J, Hadjichristodoulou C. Risk Factors for Contamination of Hotel Water Distribution Systems by Legionella Species. Appl Environ Microbiol. 2007;73(5):1489-92. Epub 2007 Jan 19. PubMed PMID: 17261527; PubMed Central PMCID:PMC1828777.
- European Centre for Disease Prevention and Control (ECDC), 2017. Legionnaires’ Disease. ECDC. Annual Epidemiological Report for 2015. ECDC, Stockholm
- Rota MC, Caporali MG, Bella A, Scaturro M, Giannitelli S, Ricci ML. Rapporto annuale sulla Legionellosi in Italia nel 2017 (Annual Report on Legionellosis in Italy-2017). Not Ist Super Sanità. 2018;31(9):7-12 Retrieved from: http://www.legionellaonline.it/notiziario%20legionellosi%20_9_2018.pdf (Last access: 03/19/2019).
- Centre for Disease Control and Prevention (CDC), 2018. Last update: 02/07/2018. Legionella (Legionnaires’ Disease and Pontiac Fever). Retrieved from. www.cdc.gov/legionella/surv-reporting.html, Accessed date: 8 February 2018.
- European Centre for Disease Prevention and Control (ECDC), 2018. Legionnaires’ disease. ECDC. Annual Epidemiological Report for 2016. ECDC, Stockholm
- Rota MC, Caporali MG, Caleo GM, Mandarino G, Scaturro M, Ricci ML. La legionellosi in Italia nel 2006. Rapporto annuale. (Legionellosis in Italy. Annual Report 2006). Not Ist Super Sanità. 2008;21(1):5-10. Retrieved from: http://www.legionellaonline.it/notiziario_set_2006.pdf (Last access: 10/19/2018).
- Linee Guida per la prevenzione ed il controllo della legionellosi 2000, Gazzetta Ufficiale della Repubblica Italiana n.103 del 5.05.2000. (Italian Guidelines for the Prevention and Control of Legionellosis 2000, G.U.R.I. n.103, 05.05.2000). Available at: http://www.gazzettaufficiale.it /do/gazzetta/serie_generale/3/pdfPaginato?dataPubblicazioneGazzetta=20000505&numeroGazzetta=103&tipoSerie=SG&tipoSupplemento=GU&numeroSupplemento=0&numPagina=1&edizione=0 or http://www.iss.it/binary/iss4/cont/050500.1144152817.pdf.
- Linee Guida recanti indicazioni sulla legionellosi per i gestori di strutture turistico-recettive e termali 2005, Gazzetta Ufficiale della Repubblica Italiana n.28 del 4.02.2005 (pp. 54–60). (Guidelines for Controlling Legionellosis in Tourist Accommodation and Thermal Structures 2005, G.U.R.I. n.28, 02.04.2005) Available at: http://www.gazzettaufficiale.it/do/gazzetta/serie generale/3/pdfPaginato?dataPubblicazioneGazzetta=20050204&numeroGazzetta=28&tipoSerie=SG&tipoSupplemento=GU&numeroSupplemento=0&edizione=0&elenco30giorni=&numPagina=54 or http://www.iss.it/binary/iss4/cont/28_02_05.1144152817.pdf.
- Bonadonna L, Briancesco R, Della Libera S, Lacchetti I, Paradiso R, Semproni M. Microbial Characterization of Water and Biofilms in Drinking Water Distribution Systems at Sport Facilities. Cent. Eur. J. Public Health. 2009; 17(2): 99–102.
- Leoni E, Legnani PP, Bucci Sabattini MA, Righi F. Prevalence of Legionella spp. in Swimming Pool Environment. Water Res. 2001;35(15):3749–3753. https://doi.org/10.1016/S0043-1354 (01)00075-6.
- De Filippis P, Mozzetti C, Messina A, D’Alò GL. Prevalence of Legionella in Retirement Homes and Group Homes Water Distribution Systems. Sci Total Environ. 2018;643:715-724. doi: 10.1016/j.scitotenv.2018.06.216. Epub 2018 Jun 26. PubMed PMID: 29957436.
- Bartram J, Cotruvo J, Exner M, Fricker C, Glasmacher A. Heterotrophic Plate Counts and Drinking-water Safety: The Significance of HPCs for Water Quality and Human Health. 2003 Switzerland/IWA Publishing, London, UK, WHO, Geneva Retrieved from. http://www.who.int/water_sanitation_health/dwq/HPCFull.pdf, Accessed date: 8 March 2018.
- Bonadonna L, Ottaviani M. (Ed.), 2007. Metodi analitici di riferimento per le acque destinate al consumo umano ai sensi del DL.vo 31/2001. Metodi microbiologici. (Reference Analytical Methods for Water for Human Consumption in Accordance with Legislative Decree 31/2001. Microbiological methods). Roma: Istituto Superiore di Sanità (Rapporti ISTISAN 07/5).
- ISO 19458, 2006. Water Quality – Sampling for Microbiological Analysis. International Organization for Standardization, Geneva, Switzerland.
- ISO 11731, 1998. Water Quality - Detection and Enumeration of Legionella. International Organization for Standardization, Geneva, Switzerland.
- De Filippis P, Mozzetti C, Messina A, D’Alò GL. Data on Legionella Prevalence and Water Quality in Showers of Retirement Homes and Group Homes in the Province of Rome, Lazio Region, Italy. Data Brief. 2018;19:2364-2373. doi: 10.1016/j.dib.2018.07.026. eCollection 2018 Aug. PubMed PMID: 30229110; PubMed Central PMCID: PMC6141508.
- DR0800 booklet, revised 2016. Legionella Latex Test. OXOID Limited, Wade Road, Basingstoke, Hampshire, RG24 8PW, UK. Available at: https://assets.thermofisher.com/TFS-Assets/MBD/Instructions/X5057C.pdf. Last access: 21-05-2018.
- UNI EN ISO 6222, 2001. Qualità dell’acqua - Valutazione quantitativa dei microrganismi vitali - Conteggio delle colonie per inoculo su terreno agarizzato (Water Quality - Quantitative Assessment of Viable Microorganisms - Counting of Colonies by Inoculation on Agarized Medium). UNI - Ente Nazionale Italiano di Unificazione.
- van der Zee A, Verbakel H, De Jong C, Pot R, Bergmans A, Peeters M, Schneeberger P, Schellekens J. Novel PCR-Probe Assay for Detection and Discrimination between Legionella pneumophila and Other Legionella Species in Clinical Samples. J. Clin. Microbiol. 2002;40 (3):1124-1125. https://doi.org/10.1128/JCM.40.3.1124-1125.2002.
- StataCorp, 2013. Stata Statistical Software: Release 13. StataCorp LP, College Station, TX
- Fragou K, Kokkinos P, Gogos C, Alamanos Y, Vantarakis A. Prevalence of Legionella spp. in Water Systems of Hospitals and Hotels in South Western Greece. Int J Environ Health Res. 2012;22(4):340-54. doi: 10.1080/09603123.2011.643229.Epub 2011 Dec 12. PubMed PMID: 22149148.
- Erdogan H, Arslan H. Colonization of Legionella Species in Turkish Baths in Hotels in Alanya, Turkey. Environ Monit Assess. 2015;187(5):235. doi: 10.1007/s10661-015-4444-3. Epub 2015 Apr 8. PubMed PMID: 25850992.
- Barna Z, Kádár M, Kálmán E, Scheirich Szax A, Vargha M. Prevalence of Legionella in Premise Plumbing in Hungary. Water Res. 2016;90: 71-78. doi: 10.1016/j.watres.2015. 12. 004. Epub 2015 Dec 10. PubMed PMID: 26724441.
- Bonetta S, Bonetta S, Ferretti E, Balocco F, Carraro E. Evaluation of Legionella Pneumophila Contamination in Italian Hotel Water Systems by Quantitative Real-Time PCR and Culture Methods. J Appl Microbiol. 2010;108(5):1576-83. doi: 10.1111/j.1365-2672.2009.04553.x. Epub 2009 Sep 11. PubMed PMID: 19796090.
- Edagawa A, Kimura A, Doi H, Tanaka H, Tomioka K, Sakabe K, Nakajima C, Suzuki Y. Detection of Culturable and Nonculturable Legionella Species Ffrom Hot Water Systems of Public Buildings in Japan. J Appl Microbiol. 2008;105(6):2104-14. doi: 10.1111/j.1365-2672. 2008. 03932.x. PubMed PMID: 19120656.
- Collins S, Stevenson D, Bennett A, Walker J. Occurrence of Legionella in UK Household Showers. Int J Hyg Environ Health. 2017;220:401-406. doi:10.1016/j.ijheh.2016.12.001. Epub 2016 Dec 2. PubMed PMID: 27964907.
- Ducret A, Chabalier M, Dukan S. Characterization and Resuscitation of ‘Nonculturable’ Cells of Legionella pneumophila. BMC Microbiol. 2014;14:3. https://doi.org/10.1186/1471-2180-14-3.
- Fields BS, Haupt T, Davis JP, Arduino MJ, Miller PH, Butler JC. Pontiac Fever Due to Legionella Micdadei from a Whirlpool Spa: Possible Role of Bacterial Endotoxin. J. Infect. Dis. 2001;184 (10):1289-1292. https://doi.org/10.1086/324211.
- Kirschner AKT. Determination of Viable Legionella in Engineered Water Systems: Do We Find What We Are Looking for? Water Res. 2016;93:276-288. https://doi.org/10.1016/j.watres.2016. 02.016.
- Alleron L, Merlet N, Lacombe C, Frère J. Long-Term Survival of Legionella Pneumophila in the Viable but Nonculturable State after Monochloramine Treatment. Curr Microbiol. 2008;57(5):497-502. doi: 10.1007/s00284-008-9275-9. Epub 2008 Oct 7. PubMed PMID: 18839249.
- Casini B, Baggiani A, Totaro M, Mansi A, Costa AL, Aquino F, Miccoli M, Valentini P, Bruschi F, Lopalco PL, Privitera G. Detection of Viable but Non-Culturable Legionella in Hospital Water Network Following Monochloramine Disinfection. J Hosp Infect. 2018;98(1):46-52. doi:10.1016/j.jhin.2017.09.006. Epub 2017 Sep 13. PubMed PMID: 28917570.
- Donati M, Cremonini E, Di Francesco A, Dallolio L, Biondi R, Muthusamy R, Leoni E. Prevalence of Simkania Negevensis in Chlorinated Water from Spa Swimming Pools and Domestic Supplies. J Appl Microbiol. 2015;118(4):1076-82. doi: 10.1111/jam.12761. Epub 2015 Feb 17. PubMed PMID: 25619531.
- Brousseau N, Lévesque B, Guillemet TA, Cantin P, Gauvin D, Giroux JP, Gingras S, Proulx F, Côté PA, Dewailly E. Contamination of Public Whirlpool Spas: Factors Associated with the Presence of Legionella spp., Pseudomonas aeruginosa and Escherichia coli. Int J Environ Health Res. 2013;23(1):1-15. doi:10.1080/09603123.2012.678001. Epub 2012 Jun 25. PubMed PMID: 22731241.
- Guillemet TA, Lévesque B, Gauvin D, Brousseau N, Giroux JP, Cantin P. Assessment of Real-Time PCR for Quantification of Legionella Spp. in Spa Water. Lett Appl Microbiol. 2010;51(6):639-44. doi: 10.1111/j.1472-765X.2010.02947.x. Epub 2010 Oct 11. PubMed PMID: 21039668.
- Leoni E, Catalani F, Marini S, Dallolio L. Legionellosis Associated with Recreational Waters: A Systematic Review of Cases and Outbreaks in Swimming Pools, Spa Pools, and Similar Environments. Int J Environ Res Public Health. 2018;15,1612. doi: 10.3390/ijerph15081612. PubMed PMID: 30061526; PubMed Central PMCID: PMC6121464.
- Hamilton KA, Prussin AJ, Ahmed W, Haas CN. Outbreaks of Legionnaires’ Disease and Pontiac Fever 2006-2017. Curr Environ Health Rep. 2018;9. doi: 10.1007/s40572-018-0201-4. PubMed PMID: 29744757.
- Baggiani A, Casini B, Totaro M, Aquino F, Valentini P, Bruni B, Porretta A, Casalini F, Miccoli M, Privitera G. Colonization by Legionella spp. of Water Networks in Residential Buildings of the Province of Pisa, Italy. Ann. Ig. 2015;27(5):718–725. https://doi.org/10. 7416/ai.2015.2064.
- Napoli C, Fasano F, Iatta R, Barbuti G, Cuna T, Montagna MT. Legionella spp. and Legionellosis in Southeastern Italy: Disease Epidemiology and Environmental Surveillance in Community and Health Care Facilities. BMC Public Health 2010;10:660. https://doi.org/10.1186/1471-2458-10-660.
- Totaro M, Valentini P, Costa AL, Frendo L, Cappello A, Casini B, Miccoli M, Privitera G, Baggiani A. Presence of Legionella spp. in Hot Water Networks of Different Italian Residential Buildings: A Three-Year Survey. Int J Environ Res Public Health. 2017;14(11):1296. doi: 10.3390/ijerph14111296. PubMed PMID: 29072607; PubMed Central PMCID: PMC5707935.
- Alary M, Joly JR. Risk Factors for Contamination of Domestic Hot Water Systems by Legionellae. Appl. Environ. Microbiol. 1991;57 (8):2360–2367.
- Falkinham JO, Hilborn ED, Arduino MJ, Pruden A, Edwards MA. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 2015;123(8):749–758. https://doi. org/10.1289/ehp.1408692.
- Mathys W, Stanke J, Harmuth M, Junge-Mathys E. Occurrence of Legionella in Hot Water Systems of Single-Family Residences in Suburbs of Two German Cities with Special Reference to Solar and District Heating. Int. J. Hyg. Environ. Health 2008;211(1–2):179–185. https://doi.org/10.1016/j.ijheh.2007.02.004.
- Zacheus OM, Martikainen PJ. Occurrence of Legionella in Hot Water Distribution Systems of Finnish Apartment Buildings. Can. J. Microbiol. 1994;40 (12): 993-999.
- Ashbolt NJ. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Health Rpt. 2015;2:95–106. https://doi.org/10.1007/s40572-014-0037-5.
- Bargellini A, Marchesi I, Righi E, Ferrari A, Cencetti S, Borella P, Rovesti S. Parameters Predictive of Legionella Contamination in Hot Water Systems: Association with Trace Elements and Heterotrophic Plate Counts. Water Res. 2011 Mar;45(6):2315-21. doi: 10.1016/j.watres.2011.01.009. Epub 2011 Jan 20. PubMed PMID: 21316728.
- Liguori G, Di Onofrio V, Gallè F, Liguori R, Nastro RA, Guida M. Occurrence of Legionella spp. in Thermal Environments: Virulence Factors and Biofilm Formation in Isolates from a Spa. Microchemical Journal. 2014;112:109-112. http://dx.doi.org/10.1016/j.microc.2013.09.023.
- Völker S, Schreiber C, Kistemann T. Drinking Water Quality in Household Supply Infrastructure: A Survey of the Current Situation in Germany. 2010;213:204-209. doi:10.1016/j.ijheh. 2010.04.005
- Solimini AG, Cottarelli A, Marinelli L, De Giusti M. Factors Influencing Persistence of Legionella Pneumophila Serogroup 1 in Laboratory Cocultures. BMC Microbiol. 2014 Oct 3;14:249. doi: 10.1186/s12866-014-0249-8. PubMed PMID: 25277877; PubMed Central PMCID: PMC4195868.
- enHealth Guidelines for Legionella Control in the Operation and Maintenance of Drinking Water Distribution Systems in Health and Aged Care Facilities (2015).
- Lucentini L, Achene L, Fuscoletti V, Nigro Di Gregorio F, Pettine P. Linee guida per la valutazione e gestione del rischio nella filiera delle acque destinate al consumo umano secondo il modello dei Water Safety Plans (Guideline for Risk Assessment and Management within the Drinking Water Chain According to Water Safety Plans). 2014 Istituto Superiore di Sanità (Rapporti ISTISAN 14/21).
- World Health Organization, 2003. Heterotrophic Plate Counts and Drinking-Water Safety: The Significance of Hpcs for Water Quality and the Human Health
- Marchesi I, Marchegiano P, Bargellini A, Cencetti S, Frezza G, Miselli M, Borella P. Effectiveness of Different Methods to Control Legionella in the Water Supply: Ten-Year Experience in an Italian University Hospital. J Hosp Infect. 2011;77(1):47-51. doi: 10.1016/ j.jhin. 2010. 09. 012. Epub 2010 Dec 4. PubMed PMID: 21131100.
- Lin YE, Stout JE, Yu VL. Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods. Infect. Control. Hosp. Epidemiol. 2011;32:166-173.
- Lin YE, Stout JE, Yu VL. Vidic RD. Disinfection of Water Distribution Systems for Legionella. Semin. Respir. Infect. 1998;13 (2):147-159. http://www.ncbi.nlm.nih.gov/pubmed/9643393
- Ji P, Rhoads WJ, Edwards MA, Pruden A. Effect of Heat Shock on Hot Water Plumbing Microbiota and Legionella pneumophila Control. Microbiome. 2018;6(1):30. doi: 10.1186/ s40168-018-0406-7. PubMed PMID: 29426363; PubMed Central PMCID: PMC5807837.
- Ortolano GA, McAlister MB, Angelbeck JA, Schaffer J, Russell RL, Maynard E, Wenz B. Hospital Water Point-Of-Use Filtration: A Complementary Strategy to Reduce the Risk of Nosocomial Infection. Am. J. Infect. Control. 2005;33 (5suppl 1) :S1-S19.
- Totaro M, De Vita E, Giorgi S, Profeti S, Porretta A, Gallo A, Frendo L, Casini B, Valentini P, Privitera G, Baggiani A. Comparison of Anolyte and Chlorine Dioxide for a Continuous Hot Water Disinfection in Nursing Home: A Two Years Legionnaires’ Disease Prevention. Journal of Water Resource and Protection, 2019; 11(3):233-243.
- Hordyjewska A, Popiołek Ł, Kocot J. The Many "Faces" of Copper in Medicine and Treatment. Biometals. 2014;27(4):611-21. doi: 10.1007/s10534-014-9736-5. Epub 2014 Apr 20. Review. PubMed PMID: 24748564; PubMed Central PMCID: PMC4113679.
- Lucaroni F, Ambrosone C, Paradiso F, Messinese M, Di Domenicantonio R, Alessandroni C, Cerone G, Cerutti F, Di Gaspare F, Morciano L, Tran L, Pietroiusti A, Palombi L. Metals Dyshomeostasis in Alzheimer’s Disease: A Systematic Review. Biomedicine & Prevention. 2017; 2 (112). DOI:10.19252/000000070
- Walraven N, Poolb W, Chapman C. Efficacy of Copper-Silver Ionisation in Controlling Legionella in Complex Water Distribution Systems and a Cooling Tower: Over 5 Years of Practical Experience. Journal of Water Process Engineering, 2016; 16:196-205.
- World Health Organization, 2004. Copper in Drinking-water - Background Document for Development of WHO Guidelines for Drinking-water Quality.
- IOM (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. A Report of the Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board, Institute of Medicine. Washington, DC, National Academy Press.
- Kelsey MC. Control of Waterborne Microorganisms and Reducing the Threat from Legionella and Pseudomonas. Decontamination in Hospitals and Healthcare. 2014. Woodhead Publishing Limited, 208-230.
- Von Gunten U. Ozonation of Drinking Water: Part II. Disinfection and By-Product Formation in Presence of Bromide, Iodide and Chlorine. Water Res. 2003; 37:1469-1487.
- Walser SM, Gerstner DG, Brenner B, Höller C, Liebl B, Herr CE. Assessing the Environmental Health Relevance of Cooling Towers - A Systematic Review of Legionellosis Outbreaks. Int J Hyg Environ Health. 2014;217(2-3):145-54. doi: 10.1016/j.ijheh.2013.08.002. Epub 2013 Sep 9. Review. PubMed PMID: 24100053.
- Outbreak news today – News Desk. Legionnaires’ Disease Outbreak in Lombardy Region, Italy. Available at: http://outbreaknewstoday.com/legionnaires-disease-outbreak-lombardy-region-italy-50916/ Last access: 10-23-2018.