A Meta-analysis of the Effect of Convalescent Plasma over Standard Treatment in Covid-19 Pneumonia
Eva Muja (Duraku),1 Ilirian Laçi,2 Sonil Marko,3 Ilir Akshija4
1 Catholic University “Our Lady of Good Counsel”, Tirana, Albania, Catholic Hospital, “Our Lady of Good Counsel”, Cardiology Department
2 Medical University of Tirana, Catholic Hospital, “Our Lady of Good Counsel”, Radiology Department
3 Medical University of Tirana, Catholic Hospital, “Our Lady of Good Counsel”, Internal Medicine Department
4 Medical University of Tirana, Statistics Department
Declaration of Interest: All authors have no conflicts of interest and no financial relationship to be disclosed.
Acknowledgements: The authors contributed equally to this work: they collected data from literature, ran the meta-analysis separately and then drafted the manuscript that was extensively revised by Dr. Akshija.
Disclosure Summary: All authors have nothing to disclose.
Data Availability Statement: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Introduction
Since late 2019, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread around the world with high rates of transmission and substantial mortality.1 COVID-19 has clinical manifestations ranging from no symptoms to respiratory failure.2 So far, some agents have shown a degree of clinical efficacy in large randomized, controlled trials like remdesivir, in hospitalized patients with pulmonary disease, and dexamethasone, in hospitalized patients receiving oxygen.3-5, 24, 25 In addition to these standards of care, health professionals have tried to manage COVID-19 revisiting older strategies, such as convalescent plasma. Convalescent plasma is a source of antiviral neutralizing antibodies. In the pre-vaccine era, convalescent plasma was used to treat viral diseases such as poliomyelitis, measles, mumps, and influenza, and, more recently, influenza, Ebola virus disease, and severe acute respiratory syndrome coronavirus epidemics, with varying degrees of success.6-8 Based on the experience of treating these viral infectious diseases, it might be worthwhile to test the safety and efficacy of convalescent plasma transfusion in SARS-CoV-2-infected patients.20-23 Although the use of convalescent plasma shows promise, the evidence supporting its use in the treatment of COVID-19 remains limited. In fact, available information stands on anecdotal cases, experience based on limited hospital data and few relatively small, randomized trials conducted after the 2019 coronavirus outbreak.9 We hypothesized that pooling the suitable studies in a meta-analysis might provide more solid information on the efficacy of this treatment and/or at least provide data that will be useful to better frame future studies.
Methods
We searched the literature for studies about the use of convalescent plasma in COVID-19 pneumonia using the search terms convalescent plasma or hyperimmune serum, COVID-19 pneumonia or SARS-CoV-2. We searched in two large databases, PubMed and Cochrane library for articles published from January to December 2020 and that met some predefined quality criteria that could be eligible for a meta-analysis following the Cochrane Methodology. We found 1255 trials in the Cochrane library and 401 on PubMed. After the title and abstract reading, we found 42 studies related to our criteria. After the final assessment of the articles, 6 of them were chosen as final studies to be included in the meta-analyses. We worked using a standardized sheet to extract information on the first author, year of publication, country of the study group, retrospective or prospective design, sample size, primary and secondary outcomes, adverse events, other medical conditions and medications for the plasma group and the control. The next step is the quality assessment by five independent professionals using a visual analogical scale for each study assessment (the analogical scale in the supplementary).
Inclusion and exclusion criteria and data extraction (in the supplementary)
Supplementary tables and data
Table 3. Wald Test, Intercept -0,232[-0,546- 0,082], p 0,147
Table 5. Egger’s test for the invasive ventilation
Endpoints
The primary endpoint is the reduction of the death rate between the arms assigned to convalescent plasma and those assigned to standard treatment alone.
The secondary endpoint is the need for invasive ventilation in both groups: the convalescent plasma group and the control group.
Statistical analysis
Statistical analysis was realized through JASP software, an open-source software based on R. Method was set based on the Omnibus Test of Model Coefficients and the Test of Residual Heterogeneity.
The effect size and the standard error were calculated according to the software producer’s recommendations. The estimated result of each study for the intervention was represented through intercept and 95% Confidence Interval. Funnel and forest plots were produced. Egger's test was performed to evaluate asymmetry.
Results
The details of the six studies included in the meta-analyses are reported in Table 1.
Table 1. Baseline characteristics of the patients of the studies considered for the meta-analysis.
Outcomes
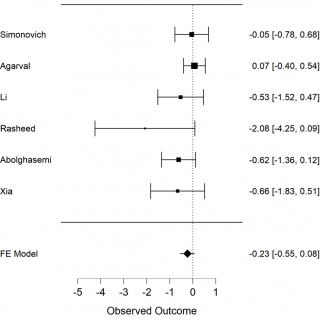
The primary outcome we analysed is death among the 2 groups in the studies, the convalescent plasma group and the control group. The secondary outcome is the necessity of invasive ventilation of the patients who received convalescent plasma in comparison with those who received only the standard treatment. We extracted the data from 6 selected studies to calculate mortality of COVID-19 patients. The results of meta-analysis of mortality are shown in forest-plot (Figure 1) and a fixed-effect model was used on the 6 studies. To assess the heterogeneity among the studies we used the Test of Residual Heterogeneity (P = 0.261). P-values above 0.100 indicate that there is no significant heterogeneity among the studies (Table 1).
Figure 1. Forest plot, mortality among groups, FE model: fixed effect model.
Table 2. Test of residual heterogeneity
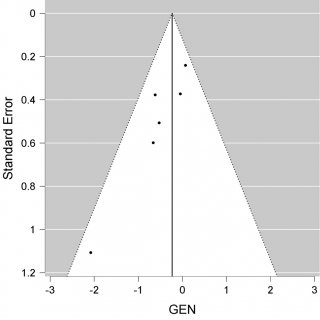
We have a non-significant result in favour of Convalescent Plasma (CP) (P = 0.147, intercept -0,232 [-0.546-0,082]). Although we may consider that this result avoids publication bias. We constructed a Funnel plot (Figure 3) to assess the asymmetry of the studies, which is an indicator of publication bias. In the Funnel plot, it seems to have a symmetric distribution of the studies, but it should be evaluated by statistics test. We used Egger’s test to assess the asymmetry, (P = 0,022), it is an indicator of bias.
Figure 3. Funnel plot of mortality.
The secondary outcome is the need for invasive ventilation. In this case we did the assessment of heterogeneity using the Test of Residual Heterogeneity (P < 0.100), which indicates that there is heterogeneity among studies. Therefore, the method used for the evaluation of the effect of plasma in invasive ventilation is the Restricted ML, a random effect model. The results of the effect of convalescent plasma on the need of invasive ventilation are shown in the Forest plot (Figure 2), (P = 0.171, intercept -1.098, CI [-2,669- 0,472]. Even though we have e result in favour of convalescent plasma in lowering the need of invasive ventilation, it is not significant (P = 0,171). We constructed a Funnel plot to assess the asymmetry (Figure 4) and also used the Egger’s test to see if it is statistically significant P = 0.274. In this case it means that there is an asymmetry and an indicator of no publication bias.
Figure 2. Forest plot of invasive ventilation need.
Figure 4. Funnel plot of invasive ventilation.
Discussion
The studies we included in the meta-analyses had different disease severity patients, from moderate to severe and life-threatening disease. This could have affected the result about the efficacy of convalescent plasma in COVID-19 and the overall result is weak in favour of convalescent plasma. This kind of result could have many reasons like the different kind of populations in the studies in terms of severity (from moderate to life threatening disease) and their different response to the treatment, the time of initiation of the treatment, the number of the participants. All the studies reported very few adverse events with the transfusion of convalescent plasma which makes plasma a safe therapeutic strategy. A review about convalescent plasma therapy reported that convalescent plasma treatment appeared effective and safe for COVID-19, but there was a need for randomized studies to further evaluate its efficacy and safety.9 Another systematic review and meta-analyses reported that convalescent plasma therapy appears safe for COVID-19, and plasma treated patients have marked reductions in their serum viral loads and most are virus negative after transfusion.10 Patients with severe COVID-19 benefit more from the convalescent plasma transfusion than critical patients,11 and patients treated in early stage are more likely to survive. Another study suggests that convalescent plasma use in severe and critically ill patients with COVID-19 may improve survival if given early in the course of disease.12 A lot of different agents have been studied since the outbreak of the pandemics. Many studies have been conducted to test the efficacy of clorochine, hydroxiclorochine, azythromycine, different antiviral agents and corticosteroids.26 Most of these medications are proven to have no effect on COVID-19 infection.13 The only agents proven in some randomized studies to have beneficial results are Remdesivir in patients with COVID-19 pneumonia and corticosteroids in those who require oxygen supplementation.3-5 Another agent, Ivermectin, has been studied initially in vitro, with good results on inhibiting the viral replication.14 A recent study published in January 2021 reported that among patients with non-severe COVID-19 and no risk factors for severe disease, receiving a single 400 mcg/kg dose of ivermectin within 72 hours of fever or cough onset resulted in no difference in the proportion of PCR-positive cases.15 The convalescent plasma was used earlier in the treatment of other infectious diseases and studying its efficacy in COVID-19 is reasonable.16-19 In summary, in our meta-analyses, convalescent plasma doesn’t have a significant effect for reducing the mortality and the need for invasive ventilation but considering it as a safe treatment, it can be worthy to continue studying its efficacy in larger studies, in order to get more solid and convincing results.
Conclusions
Convalescent plasma did not have a significant role in reducing mortality and the need for invasive ventilation compared to standard treatment in patients with COVID-19. Larger studies with a greater number of patients may have promising results.
References
- Zunyou Wu, MD, PhD, Jennifer M. McGoogan. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. Summary of a Report of 72 314 Cases from the Chinese Centre for Disease Control and Prevention. Zunyou Wu, MD, PhD Chinese Centre for Disease Control and Prevention, Beijing, China: JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
- Dawei Wang, MD; Bo Hu, MD; Chang Hu, MD; Fangfang Zhu, MD; Xing Liu, MD; Jing Zhang, MD; Binbin Wang, MD; Hui Xiang, MD; Zhenshun Cheng, MD; Yong Xiong, MD; Yan Zhao, MD; Yirong Li, MD; Xinghuan Wang, MD; Zhiyong Peng, MD. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China: doi: 10.1001/jama.2020.1585; PMID: 32031570
- J.H. Beigel, K.M. Tomashek, L.E. Dodd, A.K. Mehta, B.S. Zingman, A.C. Kalil, E. Hohmann, H.Y. Chu, A. Luetkemeyer, S. Kline, D. Lopez de Castilla, R.W. Finberg, K. Dierberg, V. Tapson, L. Hsieh, T.F. Patterson, R. Paredes, D.A. Sweeney, W.R. Short, G. Touloumi, D.C. Lye, N. Ohmagari, M. Oh, G.M. Ruiz-Palacios, T. Benfield, G. Fätkenheuer, M.G. Kortepeter, R.L. Atmar, C.B. Creech, J. Lundgren, A.G. Babiker, S. Pett, J.D. Neaton, T.H. Burgess, T. Bonnett, M. Green, M. Makowski, A. Osinusi, S. Nayak, and H.C. Lane, for the ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 — Final Report: N Engl J Med 2020;383:1813-26. N Engl J Med 2020;383:1813-26. DOI: 10.1056/NEJMoa2007764.
- Yeming Wang*, Dingyu Zhang*, Guanhua Du*, Ronghui Du*, Jianping Zhao*, Yang Jin*, Shouzhi Fu*, Ling Gao*, Zhenshun Cheng*, Qiaofa Lu*, Yi Hu*, Guangwei Luo*, Ke Wang, Yang Lu, Huadong Li, Shuzhen Wang, Shunan Ruan, Chengqing Yang, Chunlin Mei, Yi Wang, Dan Ding, Feng Wu, Xin Tang, Xianzhi Ye, Yingchun Ye, Bing Liu, Jie Yang, Wen Yin, Aili Wang, Guohui Fan, Fei Zhou, Zhibo Liu, Xiaoying Gu, Jiuyang Xu, Lianhan Shang, Yi Zhang, Lianjun Cao, Tingting Guo, Yan Wan, Hong Qin, Yushen Jiang, Thomas Jaki, Frederick G Hayden, Peter W Horby, Bin Cao, Chen Wang. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-blind, Placebo-controlled, Multicentre Trial: doi: 10.1016/S0140-6736(20)31022-9, PMID: 32423584.
- Peter Horby, F.R.C.P., Wei Shen Lim, F.R.C.P., Jonathan R. Emberson, Ph.D., Marion Mafham, M.D., Jennifer L. Bell, M.Sc., Louise Linsell, D.Phil., Natalie Staplin, Ph.D., Christopher Brightling, F.Med. Sci., Andrew Ustianowski, Ph.D., Einas Elmahi, M.Phil., Benjamin Prudon, F.R.C.P., Christopher Green, D.Phil., Timothy Felton, Ph.D., David Chadwick, Ph.D., Kanchan Rege, F.R.C.Path., Christopher Fegan, M.D., Lucy C. Chappell, Ph.D., Saul N. Faust, F.R.C.P.C.H., Thomas Jaki, Ph.D., Katie Jeffery, Ph.D., Alan Montgomery, Ph.D., Kathryn Rowan, Ph.D., Edmund Juszczak, M.Sc., J. Kenneth Baillie, M.D., Ph.D., Richard Haynes, D.M., and Martin J. Landray, Ph.D. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report The RECOVERY Collaborative Group: doi: 10.1056/NEJMoa2021436
- Thomas C. Luke, MD, MTMH Edward M. Kilbane, MD, MPH, Jeffrey L. Jackson, MD, MPH. Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1 Treatment? https://doi.org/10.7326/0003-4819-145-8-200610170-00139
- Y. Cheng · R. Wong · Y. O. Y. Soo · W. S. Wong · C. K. Lee · M. H. L. Ng · P. Chan · K. C. Wong · C. B. Leung · G. Cheng. Use of convalescent plasma therapy in SARS patients in Hong Kong. Use of Convalescent Plasma Therapy in SARS Patients in Hong Kong: doi: 10.1007/s10096-004-1271-9; PMID: 15616839.
- Noah C Peeri, Nistha Shrestha, Md Siddikur Rahman, Rafdzah Zaki, Zhengqi Tan, Saana Bibi, Mahdi Baghbanzadeh, Nasrin Aghamohammadi, Wenyi Zhang and Ubydul Haque. The SARS, MERS and Novel Coronavirus (COVID-19) Epidemics, The Newest and Biggest Global Health Threats: What Lessons Have We Learned? doi: 10.1093/ije/dyaa033; PMID: 32086938
- Binzhen Chen & Rong Xia Department of Transfusion Medicine, Huashan Hospital, Fudan University, Shanghai, China. Early Experience with Convalescent Plasma as Immunotherapy for COVID-19 in China: Knowns and Unknowns. doi: 10.1111/vox.12968; PMID: 32516839.
- Luo Wenjing1, Feng Yuanzheng, Jun-Ying Li, Liang V. Tang, Hu Yu. Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China. Safety and Efficacy of Convalescent Plasma Therapy in Severely and Critically Ill Patients with COVID-19: A Systematic Review with Metanalysis. https://doi.org/10.18632/aging.202195
- Ling Li, MD, PhD; Wei Zhang, MD; Yu Hu, MD, PhD; Xunliang Tong, MD, PhD; Shangen Zheng, MD; Juntao Yang, PhD; Yujie Kong, MD; Lili Ren, PhD; Qing Wei, MD; Heng Mei, MD, PhD; Caiying Hu, MD; Cuihua Tao, MD; Ru Yang, MD; Jue Wang, MD; Yongpei Yu, PhD; Yong Guo, PhD; Xiaoxiong Wu, MD; Zhihua Xu, MD; Li Zeng, MD; Nian Xiong, MD, PhD; Lifeng Chen, MD; Juan Wang, MD; Ning Man, MD; Yu Liu, PhD; Haixia Xu, MD; E. Deng, MS; Xuejun Zhang, MS; Chenyue Li, MD; Conghui Wang, PhD; Shisheng Su, PhD; Linqi Zhang, PhD; Jianwei Wang, PhD; Yanyun Wu, MD, PhD; Zhong Liu, MD, PhD. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19 A Randomized Clinical Trial. JAMA. 2020;324(5):460-470. doi:10.1001/jama.2020.10044.
- Livia Hegerova,1 Ted A. Gooley,2 Kelly A. Sweerus,3 Cynthia Maree,4 Neil Bailey,1 Megumi Bailey,1 Vanessa Dunleavy,1 Krish Patel,1 Kirsten Alcorn,5 Rebecca Haley,5 Jill M. Johnsen,5,6 Barbara A. Konkle,5,6 Annamarie C. Lahti,7 Morgan L. Alexander,7 Jason D. Goldman,4 Anne Lipke,3 Sun-jung Lim,3 Mark D. Sullivan,3 John S. Pauk,4 and John M. Pagel1 1 Centre for Blood Disorders and Stem Cell Transplantation, Swedish Cancer Institute, Seattle, WA; 2 Clinical Research Division, Fred Hutchinson Cancer Research Centre, Seattle, WA; 3 Division of Pulmonary and Critical Care and 4 Division of Infectious Disease, Department of Internal Medicine, Swedish Medical Centre, Seattle, WA; 5 Bloodworks Northwest, Seattle, WA; 6 Division of Hematology, University of Washington, Seattle, WA; and 7 Neuroscience Institute, Swedish Medical Centre, Seattle, W. Use of Convalescent Plasma in Hospitalized Patients with COVID-19: Case Series. DOI 10.1182/blood.2020007079.
- Ramy MohamedGhazy, AbdallahAlmaghraby, Ramy Shaaban, Ahmed Kamal, Hatem Beshir, Amr Moursi, Ahmed Ramadan, Sarah Hamed N.Taha. A Systematic Review and Meta‑analysis on Chloroquine and Hydroxychloroquine as Monotherapy or Combined with Azithromycin in COVID‑19 Treatment. | https://doi.org/10.1038/s41598-020-77748-x
- Leon Calya, Julian D. Drucea, Mike G. Cattona, David A. Jansb, Kylie M. Wagstaffb,∗ a Victorian Infectious Diseases Reference Laboratory, Royal Melbourne Hospital, At the Peter Doherty Institute for Infection and Immunity, Victoria, 3000, Australia b Biomedicine Discovery Institute, Monash University, Clayton, Vic, 3800, Australia. The FDA-approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 In Vitro. https://doi.org/10.1016/j.antiviral.2020.104787
- Carlos Chaccoura, Aina Casellasa, Andres Blanco-Di Matteo, Inigo Pineda, Alejandro Fernandez-Montero, Paula Ruiz-Castilloa, Mary-Ann Richardson, Mariano Rodríguez-Mateos, Carlota Jordan-Iborra, Joe Brew, Francisco Carmona-Torreb, Miriam Giraldez, Ester Laso, Juan C. Gabaldon-Figueira, Carlota Dobano, Gemma Moncunilla, Jose R. Yuste, Jose L. Del Pozo, N.Regina Rabinovicha, Verena Schoning, Felix Hammannh, Gabriel Reina, Belen Sadab, Mirian Fernandez-Alonso. The Effect of Early Treatment with Ivermectin on Viral Load, Symptoms and Humoral Response in Patients with Non-severe COVID-19: A Pilot, Double-blind, Placebo-controlled, Randomized Clinical Trial. DOI:https://doi.org/10.1016/j.eclinm.2020.100720
- Manuel Rojas, Yhojan Rodríguez, Diana M. Monsalve, Yeny Acosta-Ampudia, Bernardo Camacho, Juan Esteban Gallo, Adriana Rojas-Villarraga, Carolina Ramírez-Santana, Juan C. Díaz-Coronado, Rubén Manrique, Ruben D. Mantilla, Yehuda Shoenfeld, Juan-Manuel Anay. Convalescent Plasma in Covid-19: Possible Mechanisms of Action. doi: 10.1016/j.autrev.2020.102554, PMID: 32380316.
- Boping Zhou, M.D., Ph.D. Shenzhen Donghu Hospital Shenzhen 518020, China Nanshan Zhong, M.D. Guangzhou Institute of Respiratory Disease Guangzhou 510120, China Yi Guan, M.D., Ph.D. University of Hong Kong Hong Kong SAR, China. Treatment with Convalescent Plasma for Influenza A (H5N1) Infection. N Engl J Med 2007; 357:1450-1451 DOI: 10.1056/NEJMc070359
- J. van Griensven, T. Edwards, X. de Lamballerie, M.G. Semple, P. Gallian, S. Baize, P.W. Horby, H. Raoul, N. Magassouba, A. Antierens, C. Lomas, O. Faye, A.A. Sall, K. Fransen, J. Buyze, R. Ravinetto, P. Tiberghien, Y. Claeys, M. De Crop, L. Lynen, E.I. Bah, P.G. Smith, A. Delamou, A. De Weggheleire, and N. Haba, for the Ebola-Tx Consortium. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med. 2016 January 07; 374(1): 33–42. doi:10.1056/NEJMoa1511812.
- Hassan Abolghasemi, Peyman Eshghi, Abdol Majid Cheraghali, Abbas Ali Imani Fooladi, Farzaneh Bolouki Moghaddam, Sina Imanizadeh, Matin Moeini Maleki, Mohammad Ranjkesh, Mohammad Rezapour, Ali Bahramifar, Behzad Einollahi, Mohammad Javad Hosseini, Nematollah Joneidi Jafari, Mohamad Nikpouraghdam, Nariman Sadri, Mokhtar Tazik, Shanaz Sali, Shamsi Okati, Elham Askari, Payam Tabarsi, Jafar Aslani, Ehsan Sharifipour, Mohammad Hossein Jarahzadeh, Nastaran Khodakarim, Mahmood Salesi, Ramezan Jafari, Samira Shahverdi. Clinical Efficacy of Convalescent Plasma for Treatment of COVID-19 Infections: Results of A Multicentre Clinical Study. doi.org/10.1016/j.transci.2020.102875
- V.A. Simonovich, L.D. Burgos Pratx, P. Scibona, M.V. Beruto, M.G. Vallone, C. Vázquez, N. Savoy, D.H. Giunta, L.G. Pérez, M..L. Sánchez, A.V. Gamarnik, D.S. Ojeda, D.M. Santoro, P.J. Camino, S. Antelo, K. Rainero, G.P. Vidiella, E.A. Miyazaki, W. Cornistein, O.A. Trabadelo, F.M. Ross, M. Spotti, G. Funtowicz, W.E. Scordo, M.H. Losso, I. Ferniot, P.E. Pardo, E. Rodriguez, P. Rucci, J. Pasquali, N.A. Fuentes, M. Esperatti, G.A. Speroni, E.C. Nannini, A. Matteaccio, H.G. Michelangelo, D. Follmann, H.C. Lane, and W.H. Belloso, for the PlasmAr Study Group. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med 2021;384:619-29. DOI: 10.1056/NEJMoa2031304
- Anup Agarwal, Aparna Mukherjee, Gunjan Kumar, Pranab Chatterjee, Tarun Bhatnagar, Pankaj Malhotra, on behalf of the PLACID Trial Collaborators. Convalescent Plasma in the Management of Moderate Covid-19 in Adults in India: Open Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial). doi.org/10.1136/bmj.m3939
- Xinyi Xia, Kening Li, Lingxiang Wu, Zhihua Wang, Mengyan Zhu, Bin Huang, Jie Li, Ziyu Wang, Wei Wu, Min Wu, Wanlin Li, Lu Li,Yun Cai, Bakwatanisa Bosco, Aifang Zhong, Xiong Liu, Tangfeng Lv, Zhenhua Gan, Guang Chen, Yunhu Pan, Caidong Liu, Kai Zhang, Xiaoli Xu, Changjun Wang and Qianghu Wang. Improved Clinical Symptoms and Mortality Among Patients with Severe or Critical COVID-19 after Convalescent Plasma Transfusion. doi: 10.1182/blood.2020007079, PMID: 32573724
- Anwar M. Rasheed, Dhurgham F. Fatak, Hashim A. Hashim, Mohammed F. Maulood, Khulood K. Kabah, Yaqoob A. Almusawi, Ahmed S. Abdulamir. The Therapeutic Potential of Convalescent Plasma Therapy on Treating Critically-ill COVID-19 Patients Residing in Respiratory Care Units in Hospitals in Baghdad, Iraq. PMID: 32920571
- Alessandro Libra,1,* Nicola Ciancio,1 Gianluca Sambataro,1 Enrico Sciacca,1 Giuseppe Muscato,1 Andrea Marino,2 Carlo Vancheri1 and Lucia Spicuzza.1 Use of Remdesivir in Patients Hospitalized for COVID-19 Pneumonia: Effect on the Hypoxic and Inflammatory State
- Luís Coelho,1,2* Fatima Falcão,3,4 Pedro Póvoa,1,2,5, Erica Viegas,3,4 Antonio Pais Martins,6 Eduarda Carmo,7 Candida Fonseca,2,8 Luis Campos,2,8 Kamal Mansinho,2,9 Inês Carmo,3 Joana Soares,3 Mariana Solano,3 Dina Mendes,3 Ana Cláudia Miranda, 9 Antonio Carvalho, 8,10 Ana Mirco, 4,10 Helena Farinha, 4,10 Isabel Aldir2,9,10 & José Correia.10 Remdesivir and Corticosteroids in the Treatment of Hospitalized COVID‑19 Patients
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/.